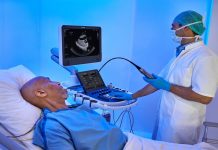
Visura Technologies, a Minneapolis-based medical device company, has unveiled the TEECAD mini, the latest advancement in transesophageal echocardiography (TEE) imaging technology. Expanding upon the success of its FDA-cleared TEECAD System, Visura is looking to further develop this exclusive technology to be compatible with smaller probes. This initiative targets adult patients whose anatomical needs are not adequately served by standard TEE probe sizes. At the American Society of Echocardiography (ASE) 2024, Visura will gather insights from key opinion leaders and demonstrate the TEECAD System's benefits at the TEECAD Innovation Station.
The TEECAD mini-program marks significant progress for Visura, following an 85% increase in evaluations at major medical centers and the expansion of its clinical and commercialization teams. Dr. David Marmor, Visura’s founder and chief medical officer, emphasized the lack of significant updates in TEE probe technology since the 1970s and highlighted the critical advancement represented by TEECAD. Clinical data, presented at last year's ASE meeting by Dr. Sunil Mankad of Mayo Clinic, demonstrated TEECAD's safety and effectiveness in esophageal imaging, which is proven to reduce probe placement time and promote first-pass intubation success, without adverse events. Visura continues to innovate and expand its product portfolio to meet market needs and improve patient care.