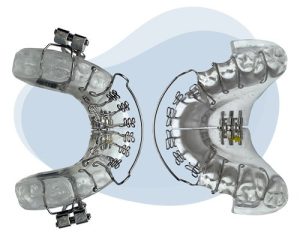
Vivos Therapeutics, Inc., a medical technology company focused on developing innovative treatments for patients suffering from dentofacial abnormalities and/or mild-to-moderate obstructive sleep apnea (OSA) and snoring in adults, recently announced a brand new clearance from the U.S. Food and Drug Administration (FDA) for its proprietary DNA appliance (daytime-nighttime appliance).
The appliance has heretofore been marketed as an orthodontic treatment for jaw expansion and teeth positioning in adults as well as children, and will continue to be available for those purposes. Its preexisting presence in clinics will make its ease of adoption a cinch; its use for palatal expansion has made it the company’s longest standing appliance in wide use among Vivos-trained dentists. The new approval for the DNA appliance as a Class II device represents an entirely unique treatment regimen for mild-to-moderate OSA.
The Vivos Method surpasses other treatments by addressing root causes of snoring/OSA in patients, training the tongue to rest in the proper position while opening up the airway via palate expansion. This effectively transitions patients from oral breathing to less obstructive nasal breathing. Moreover, the appliance has flexibility for use in connection with other treatment modalities such as CPAP or myofunctional therapy. Parts of the data submitted for the FDA’s clinical review indicated that 86% of patients had their airway size improved, and 97% saw increased width of their palate.