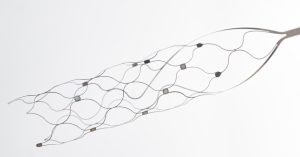
Endovascular brain interface company Synchron has announced completion of the U.S.’s very first in-human brain-computer interface (BCI). Bypassing the need for invasive open-brain surgery, the implant is indeed the first of its kind in the nation leveraging an endovascular BCI approach. The procedure was performed at Mount Sinai West in New York, with the Icahn School of Medicine’s Dr. Shahram Majidi at the helm in the angiography suite. “This is an incredibly exciting milestone for the field, because of its implications and huge potential,” Majidi said. “The implantation procedure went extremely well, and the patient was able to go home 48 hours after the surgery.”
This was the pioneering patient implant in the company’s Command trial, which falls under an FDA investigational device exemption to evaluate the prospect of a permanently implanted BCI. Command is meant to gauge the safety and efficacy of Synchron’s motor BCI technology in severe paralysis patients. The ultimate goal is to give patients control over hands-free digital devices.
The implant is outpacing Elon Musk’s Neuralink, which has yet to earn an oft-promised FDA approval for human trials. That program has been the subject of much controversy, with accusations of “egregious violations of the Animal Welfare Act” by the Physicians Committee for Responsible Medicine (PCRM). Allegations included extreme suffering for monkeys subjected to testing.