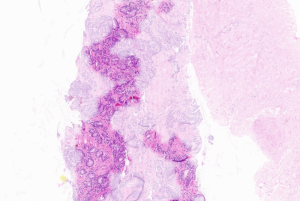
Clinical Pathology AP application developer Paige has earned its first European regulatory certifications for a benchmark offering. The company recently scored UKCA and CE-IVD marks for its Prostate Biomarker Suite. On digitized tissue images stained with hematoxylin and eosin, the AI can identify the existence of four biomarkers for prostate cancer. The U.S. has not cleared the software for use in diagnostic procedures, limiting its availability to Research Use Only.
The aforementioned biomarkers, linked to prostate cancer development and progression, are Androgen Receptor (AR), TP53, RB1, and PTEN. They aid physicians in stratifying patients into treatment paradigms as well as help to facilitate targeted clinical trial enrollment. Paige developed the Biomarker Suite using the foundational technology containing histology image data collated from scores of patients that powered its Prostate Detect product, which has earned CE-IVD and UKCA marks as well as FDA approval.
“By employing Paige Prostate Biomarker Suite, clinicians can rapidly reduce laboratory turnaround time while providing a broader range of data at the point of diagnosis,” said Paige President and Chief Business Officer Dr. Jill Stefanelli. “We’re excited by this regulatory milestone of our biomarker capabilities built on our robust AI technology platform, which can rapidly screen and develop proof-of-concept biomarkers.” Image-based biomarkers, as opposed to the more common molecular biomarker testing methodology, can speed result times, foster useful information at point of diagnosis, and provide extra protection for sensitive tissue samples.