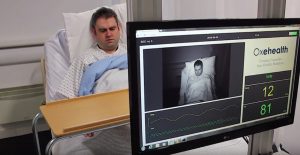
A new video-monitoring software developed at Oxford University has recently received De Novo clearance by the FDA. A year after its initial submission the Oxehealth platform has been given the go-ahead to enter the U.S. market.
This software utilizes video camera signals to measure heart rates, pulse rates, breathing frequency, and respiratory movement by running video streams through a specialized algorithm that identifies pixel-level changes which correlate with measuring these vitals. In application, the Oxehealth Vital Signs device will be a fixed installation in single-occupancy rooms that can be used in a variety of settings depending on the healthcare professional’s needs.
This software offers a completely non-invasive approach, which would be helpful in application at places like mental health facilities, where sometimes no direct contact is ideal in elevated situations. In clinical trials, the system’s optical sensors performed within three beats per minute of a standard oximeter reading and could count breaths by monitoring movements in the chest wall.
According to Oxford’s medical device disclaimer page, the software is only meant for use in individuals aged 18 and over, who do not need critical care or continuous monitoring of their vitals. The software also has some non-medical uses including activity and movement tracking, fall risk, and sleep-related applications.
In 2018 the technology was deemed a Class iia medical device by EU regulators. The device has already been deployed to multiple locations all over the U.K. including mental health hospitals and police forces, where they are piloting the technology in local prisons. The remote applications of this software are also able to assist in the COVID-19 age, where contactless monitoring offers a much-needed safety barrier.
The company has noted that in 2020 alone, annual revenue doubled, and has announced that Oxehealth will be seeking a collective £10 million in two rounds spanning between 2021 and 2022, in order to expand their platform and begin further catering to its newly acquired market.
The long-term applications of this type of technology could greatly increase the safety of both patients and healthcare professionals. With fine tuning, this software is bound to become advanced enough to become a viable replacement for many vital-monitoring methods that are traditionally used.