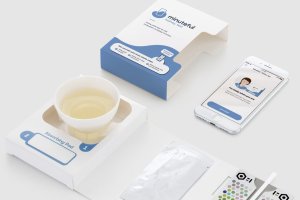
Adding yet another innovation leveraging smartphone camera technology for medical device capabilities to its extensive portfolio, Healthy.io has picked up 510(k) clearance from the FDA for home use of its Minuteful Kidney™ test. This historic approval is the first time the agency has given the green light to a smartphone-powered home test for evaluation of albumin to creatinine ratio (ACR).
Prior to the landmark clearance, the test was only available if administered by a medical practitioner. Accordingly, some 60 million Americans, which account for around 80% of patients at risk for Chronic Kidney Disease (CKD), do not complete a highly recommended annual test. Now, using only a smartphone camera, individuals can conduct a home test assessing albumin levels in urine—the standard of care for CKD detection.
"We have the experience, results, and regulatory assurances to fundamentally change how Americans monitor chronic conditions using their smartphone camera", said Healthy.io’s Founder and Chief Executive Officer, Yonatan Adiri. "In effect, Minuteful Kidney™ is a digital antidote that can help every person at-risk dramatically decrease their probability of undergoing dialysis.”
Currently, Medicare is shelling out roughly $120 billion annually to treat Chronic Kidney Disease and End Stage Renal Disease (ESRD). With one in three Americans at risk of a “silent killer” condition that doesn’t present noticeable symptoms until late stages, diagnostic and turnaround solutions for CKS are hot-ticket items.