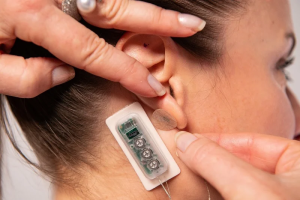
DyAnsys’ First Relief PENS (percutaneous electrical neurostimulation) device has been granted FDA approval for multiple treatments for up to 56 days for the relief of chronic diabetic peripheral neuropathy-related pain. This wearable device, which attaches to the ear, emits continuous pulses of low-level electrical current over a period of several days. The FDA’s thumbs-up was predicated on a study that assessed First Relief in comparison to a placebo and another similar device that had also been granted an FDA clearance.
"We are excited to have the FDA clearance of First Relief so that this device, which has been proven effective, can now be used to treat patients who have been experiencing pain related to diabetic neuropathy,” said Srini Nageshwar, Chief Executive Officer of DyAnsys. "First Relief offers a significant treatment option without drugs or narcotics."
Jeevak Multispeciality Hospital in Warangal, India, which is famous for its diabetes treatment undertakings, hosted the single center, three-arm, randomized, controlled, parallel assignment, double blinded, prospective study. The devices were applied on a bi-weekly basis for four months on 63 patients ages 30 to 74, with the main efficacy endpoint being pain intensity as measured through Visual Analog Scale score. Secondary efficacy endpoints included vibration perception threshold (VPT) value, insomnia severity index (ISI), overall neuropathy limitations scale (ONLS), and the Hamilton rating scale for anxiety. During the study period, no complications or adverse events were observed in any of the subjects.