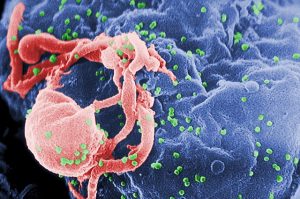
Merck has caught a lucky break with its HIV treatment solutions enterprise, as the FDA has cleared its drug islatravir of a nine-month clinical trial hold. The only condition of the release: low doses are used. Merck has also indicated it will no longer spend time exploring islatravir as a preventative treatment.
The FDA had issued its hold due to the drug’s potential for decreasing total lymphocyte and CD4+ T-cell counts in individuals receiving the therapy. Similar concerns also caused Merck to call a halt on trials for its other HIV asset in the running, MK-8507. Now, with a green light from the agency, Merck is embarking on three new phase-3 trials that will use a 0.75-mg dose of the aforementioned drug in tandem with its previously approved HIV drug Pifeltro. Two of the trials will look for results from virologically suppressed adults with HIV-1 infection, while the other will pertain to previously untreated adults with HIV-1 infection. It’s safe to say that any studies using higher doses of islatravir won’t see the light of day any time soon. In fact, the lower dose will allow for a separate phase-2 trial using a combination regiment of the drug and Gilead Sciences' lenacapavir.