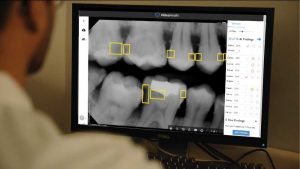
The FDA has acted as a sort of tooth fairy by granting a 510(k) clearance to Massachusetts-based VideaHealth’s artificial algorithm that assists dentists with cavity identification in their patients’ X-rays. The Videa Factory, the company’s comprehensive dental X-ray database, was instrumental in building and training this advanced AI tool. VideaHealth posits that its Factory holds the most diverse data set in the industry, comprising over 100 million data points taken from top insurance organizations, dental service companies, universities, and clearinghouses.
Helping to ensure a positive decision, VideaHealth supplied the FDA with some promising clinical trial results. The AI in question was able to reduce the amount of unnoticed carious lesions by dentists by 43%. Moreover, incorrect diagnoses were mitigated by roughly 15%. “Our biggest priority as a team is ensuring that our solution is effective across diverse patient populations and helps dentists deliver the most accurate diagnoses,” said VideaHealth Chief Executive Officer and Founder Florian Hillen. “This paves the way for more appropriate dental treatment recommendations and the opportunity for dentists to foster deeper patient engagement.”
VideaHealth’s FDA win follows the recent close of a lucrative Series A funding round led by Spark Capital – Pillar VC and Zetta Venture Partners, two existing investors, also contributed. The $20 million raise from the end of March lifts its lifetime fundraising total to $26.4 million. Foreshadowing the recent FDA clearance, the company had at that time indicated a desire to funnel the cash to “massively expand” its AI-powered diagnostic undertakings, hoping to reach more dentists across the U.S. and aiming for coverage of around 3.5% of all practices in the nation.