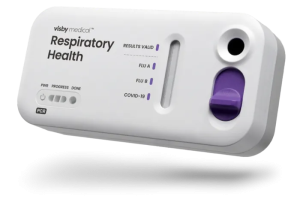
Diagnostics innovator Visby Medical has been granted an Emergency Use Authorization (EUA) from the FDA for its point of care Respiratory Health Test. A quick-acting PCR device that differentiates between upper respiratory infections triggered by Influenza A & B as well as COVID-19, the test can fit in the palm of the hand and produces accurate results in less than a half hour. At present, PCR tech is the pinnacle of testing for the aforementioned viruses.
Visby’s advantage in this space is its pioneering “instrument-free” platform. The Infectious Disease Society of America and the CDC concur in recommending clinicians take advantage of rapid molecular assays if possible, as they are preferential over rapid influenza diagnostic tests (RIDTs) in improving detection rates in outpatients. Expense has been a major barrier in the potential widespread adoption of PCR testing, however.
Visby’s solution, which has been bolstered by federal funds from the Department of Health and Human Services, the Administration for Strategic Preparedness and Response, and the Biomedical Advanced Research and Development Authority (BARDA), promises to make it a market mainstay.
"Accurate, rapid, point of care testing can help physicians prescribe appropriate antiviral medications, minimize viral transmission in waiting rooms, and improve patient satisfaction," said Visby Medical’s Chief Medical Officer, Dr. Gary Schoolnik. "Use of the Visby Respiratory Health Test at the point of care in urgent care clinics and emergency rooms will transform how patients with respiratory symptoms are diagnosed and treated."