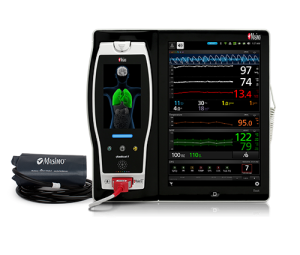
Medtech company Masimo, which specializes in noninvasive patient monitoring technologies, has nabbed an expanded FDA clearance for the use of its SedLine brain function monitoring system and sensors. In the U.S., the system is now approved for use as an anesthetic effectiveness assessment tool on patients as young as one year old.
The FDA originally approved SedLine in 2004, and since then, the system has undergone numerous technological updates. All versions of the devices were approved for the monitoring of brain activity of adult patients under anesthesia.
Masimo is also known for its SET pulse oximeter and the CE-marked Rainbow SuperSensor, which continuously aggregates readings of 12 health parameters. The company is wasting no time capitalizing on the FDA’s bigger thumbs up; a version of the system specifically developed for pediatric cases is already available. One main adjustment is the downsizing of its noninvasive electrode sensors to better fit the forehead of a child.
“SedLine is achieving for brain function monitoring what Masimo SET did for pulse oximetry,” said Joe Kiani, Masimo’s Founder and Chief Executive Officer. “We believe SedLine is the best and most advanced way to monitor depth of sedation, crucial to helping ensure patients with even the most challenging and the youngest brains are appropriately anesthetized.”
As 2021 drew to a close, Masimo made public the results of a prospective study that indicated SedLine technology may be able to help identify risk of developing anesthesia-related conditions during and after surgery for adult patients.