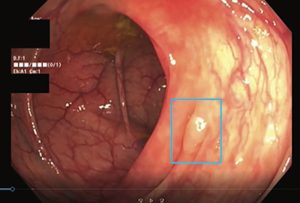
The FDA has granted 510(k) clearance to polyp detection device SKOUT from gastroenterology precision medicine tech maker Iterative Scopes and Provation, a leading software and SaaS provider of clinical automation solutions. The computer-guided system uses technology developed to spot suspicious tissue and provide guidance and feedback to GI physicians during assessments. SKOUT was proven to drastically improve overall adenoma detection in screening and surveillance colonoscopies compared to standard colonoscopies by a U.S.-based multicenter clinical study for computer-aided polyp detection (CADe), which happens to be the largest study of its kind conducted to date.
“Even among the best endoscopists, there is room for improvement in adenoma detection, which can impact patient outcomes,” said Iterative Scopes’ Vice President of Clinical Operations, Sloane Allebes Phillips. “We are enthusiastic about the fact that even gastroenterologists with an already high baseline rate of adenoma detection demonstrated an improvement with SKOUT. Now that SKOUT is FDA-cleared, clinicians will be able to better detect adenomas with more efficiency, and ultimately change the standard of gastrointestinal care.”
The company first teamed with Provation early last year to hone a series of AI-based gastrointestinal documentation solutions. Now, Provation will take charge of exclusive distribution of SKOUT, as the two companies look to broaden the system’s availability in top U.S. GI organizations.
The second leading cause of cancer deaths in the U.S and Europe, colorectal cancer is so often fatal in part because of a high number of undiagnosed adenomas—endoscopists miss them at a rate of 26% during colonoscopies. SKOUT’s registration trial found the technology significantly improved adenoma detection, as measured by adenomas per colonoscopy (APC).