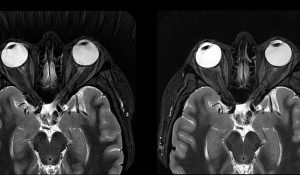
The FDA has granted its coveted 510(k) clearance to GE Healthcare’s new AIR Recon DL for 3D and Propeller imaging sequences. The upsides of the solution pertain to the entire gamut of MRI clinical procedures, covering all anatomies as well as improving image quality, scan times, and the overall patient experience.
Moving beyond 2D, the AIR Recon DL platform’s 3D capabilities give physicians extra help in leveraging signal-to-noise (SNR) ratio and sharpness for more accurate and streamlined diagnostic processes. “By expanding AIR Recon DL to 3D and Propeller, GE Healthcare has closed the gaps in our ability to provide improved image quality and patient experience to all our patients across exam types, particularly for brain imaging, where we rely heavily on 3D sequences and musculoskeletal imaging, where Propeller is important for reducing image quality variability and eliminating repeat sequences due to motion,” said Dr. Tiron Pechet, a Shields Health Care Group radiologist and assistant medical director.
A recent study from GE Healthcare saw the entirety of its participants note improved SNR ratio and image sharpness. Though falling short of that impressive 100% figure, 99% of the study group also expressed that AIR Recon DL brings better or at least equivalent lesion conspicuity. The same report concluded that the solution can result in a 50% reduction in exam times. Its compatibility with Propeller motion-insensitive imaging sequencing make it a crucial add-on for physicians diagnosing and treating pediatric, neurodegenerative, geriatric, and claustrophobic patients.