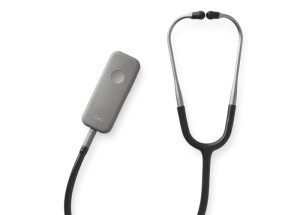
The innovative stethoscope solutions company Eko has scored an FDA approval for its Eko Murmur Analysis Software (EMAS) for adult and pediatric patients. Having already risen to the top of the murmur detection capability market, Eko’s EMAS is a first-of-its-kind AI-powered smart stethoscope that can, within mere seconds, identify as well as discern between the innocent and structural heart murmurs that are potential tell-tale indications of valvular heart disease. The company has additionally entered into a partnership with 3M to connect its AI and software algorithms to offer clinicians access to 3M’s own stethoscope for similar purposes.
“This latest FDA clearance is another way in which Eko is improving access to better heart health through clinically-validated algorithms and best-in-class medical devices,” said Connor Landgraf, Eko’s Co-Founder and Chief Executive Officer. “By making heart disease screening algorithms and digital stethoscopes accessible in exam rooms around the country, we are moving towards a future in which more objective and consistent valvular heart disease screening can become the standard of care.”
The EMAS algorithm has produced drastically improved performance standards in its relatively short clinical use lifetime, with healthcare professionals spotting valvular heart disease at an overall sensitivity of 85.6% and a specificity of 84.4%. Analysis shows that, in adults over 18 years old, structural murmur identification can even reach a sensitivity of 90.2% and specificity of 90.6%. This represents a sizable advancement from the results achievable with traditional stethoscopes, which detect with a sensitivity of 44% and specificity of 69%.