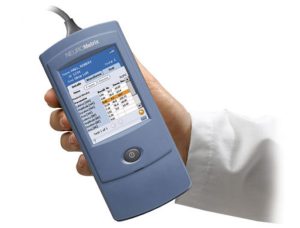
NeuroMetrix, a Massachusetts-based nerve conduction technology developer, has announced the commercial launch of its DPNCheck 2.0 point-of-care (POC) device. The innovation was designed to detect peripheral neuropathy using nerve conduction technology, providing accelerated print screening with quantitative measurement of peripheral nerve function. DPNCheck 2.0 represents a significant advancement in peripheral neuropathy testing, which traditionally has involved monofilament and tuning forks.
Coming with a convenient and intuitive touchscreen, guided onboarding instructions, and improved temperature compensation, DPNCheck 2.0 also gives the user real-time nerve response displays. Moreover, all clinical documentation of test results are handled by a companion software. NeuroMetrix has indicated that it will work with provider EHR systems on integration of data this year, and plans to tap into cloud technology for aggregation of population health data.
“For more than a decade, our first-generation DPNCheck device has been used and trusted by thousands of providers to assess over 2 million patients,” said the company’s President, CEO, and Chairman of the Board, Dr. Shai N. Gozani. “We’ve opened the door to more widespread screening with a user-friendly design that makes it faster and easier for care organizations to roll out and train their point-of-care staff and deploy testing.”