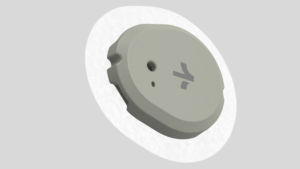
Dexcom has received clearance from the U.S. Food and Drug Administration for its new over-the-counter continuous glucose monitor (CGM) named Stelo. Unveiled on March 5, 2024, Stelo is designed for Type 2 diabetes patients who do not use insulin, making it the first glucose biosensor of its kind that does not require a prescription. The device, set to be available for online purchase this summer, offers real-time glucose level tracking through small sensors worn on the upper arm. Unlike Dexcom's existing G7 CGM system, which requires a prescription, Stelo aims to provide a simpler experience with a unique platform tailored specifically to the needs of Type 2 diabetes patients.
With over 25 million Type 2 diabetes patients in the U.S. not using insulin, Dexcom's Stelo aims to expand access to CGMs by allowing individuals to purchase the device without the involvement of a healthcare provider. Dexcom's Chief Operating Officer, Jake Leach, emphasized that Stelo's platform will prioritize simplicity, omitting certain alerts and notifications meant for patients at risk of more serious emergencies. While initially offered at an "approachable" cash pay price, Dexcom believes that as the benefits of Stelo are demonstrated, insurance companies will eventually cover its cost, providing users with a valuable and personal insight into their glucose levels.