Silicon Valley-based medical device company Anitoa Systems is augmenting its SARS-CoV-2 rapid nucleic acid test portfolio by introducing two kits capable of detecting the virus' Omicron and Delta mutations. Anitoa is now ready to put its research-use-only SARS-CoV-2, Delta, and Omicron kit and the CE-IVD certified SARS-CoV-2 and Delta variant kit at the disposal of health systems and patients.
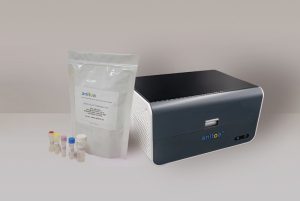
Anitoa Systems Chief Executive Officer Dr. Zhimin Ding said, “As a key supplier of equipment and materials for detecting SAR-CoV-2, Anitoa is closely monitoring all current and emerging variants of this deadly virus to ensure that our test solutions are relevant to the need of the people combating this disease.” Anitoa has incorporated Maverick, an in-house portable real-time polymerase chain reaction (qPCR) instrument, and its reverse transcription qPCR (RT-qPCR) reagent in order to test for SARS-CoV-2 and its variants.
This solution is a major victory for testing turnaround and convenience. It bypasses the often convoluted processes associated with packing and shipping patient samples to a central laboratory in addition to mitigating the need for extended RT-qPCR prep time. Anitoa noted that not only does this protocol save time, but it greatly reduces the risk of cross-contamination.
Moreover, prolonged transportation leaves the viral RNA of samples vulnerable to degradation. Anitoa's flexible device lessens the chance of false-negative readings by doing away with freezing, thawing, and the need for transit while allowing samples in the form of nasal or throat swabs to be applied directly to the instrument.