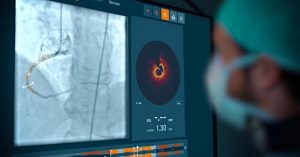
Healthcare and medical devices company Abbott has achieved FDA clearance for its Ultreon 1.0 imaging software. This AI-powered innovation affords physicians improved clarity on blood flow, blockages, and other heart-related surveillance factors. Ultreon 1.0 leverages its AI engine with optical coherence tomography to deliver more informed decision-making opportunities for healthcare professionals.
Traditional imaging methods are increasingly being abandoned, as emerging technologies have revolutionized cardiology assessments. Ultreon 1.0 can gauge calcium levels and vessel diameters with great accuracy to help physicians perform effective and prompt stent placement.
The software has earned a CE Mark clearance in Europe, and was implemented for London’s Royal Free Hospital in May.
In one week, Abbott has doubled up on FDA clearances; its FreeStyle Libre 2 was also given the go-ahead. That technology lets diabetic patients forgo a reader when accessing their glucose levels on iPhones.
The exciting prospects that AI technology has generated in healthcare of late, as well as its significance in COVID-19 treatment, ensure that it will play an increasingly-important role in the sector. A survey from care service group Optum found that 83% of healthcare organizations have integrated an AI gameplan, and 15% had strong desires to establish one. 59% of executives in the sector projected a return on their AI investments within 3 years. A mere 31% were of the same opinion circa 2018.